Electricity is one of many forms of energy, and energy has the ability or potential to do work. The potential to do work constitutes the usefulness of energy. For example, electrical energy causes light bulbs to glow, electrical motors to spin, electrical stove burners to generate heat, spark plugs to create sparks, and electrical signals to be transmitted wirelessly through space and around the world. Energy in general, and electrical energy specifically, can be transformed into different forms of energy. Light, force, heat, magnetic waves, and radio frequencies are all common forms of energy. Energy transformation is a spectacular phenomenon. The essence of energy can be expressed in a variety of different ways, one of which will be the focus of this article: electrical energy. Electrical energy is extremely beneficial and useful. However, it can also be equally perilous and lethal, considering the electrical systems on electric and hybrid vehicles. As automotive instructors, how can we introduce such a phenomenon to students while allowing them to identify, understand, and respect the function, application, and dangers of electricity?
Electricity Made Simple - Part 1
Good Foundation For Understanding Electrical Theory
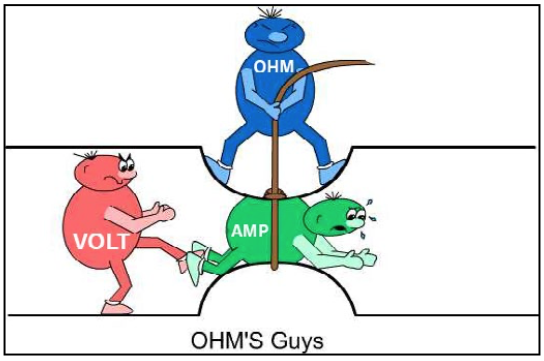
This article is my perspective on one of the most important subjects in automotive service technology. Helping automotive students develop a good foundation for understanding electrical theory and its application is essential in learning effective automotive electrical troubleshooting and diagnostic skills. Automotive students are often challenged and overwhelmed by the study of electrical theory and Ohm’s Law. Ohm’s Law is a scientific/ mathematical formula introduced by George Simone Ohm (1789- 1854; German physicist) to express the relationship between voltage, current, and resistance. Although Ohm’s Law captures the mathematical relationship between these electrical terms, it is only a fraction of numerous combinations of electrical formulas used in basic and advanced electrical calculations. Visualizing and experiencing the principles and dynamics of electricity is the key to developing a good foundation for students to learn electrical theory.
The fundamental questions students should be encouraged to focus on are: What is electricity? and How does electricity come into being? I encourage students to grasp the concept of electricity from this perspective. After 20 years or more of teaching, I still enjoy the fun and excitement of attempting to explain how an invisible concept such as energy is responsible for countless visible and tangible experiences in and outside the realm of automotive service and technology. This challenge adds tremendous value to the joy, effort, and skill of teaching experiences. Teaching electrical theory is like explaining the dynamics, function, and benefits of air. Air is not visible, yet we cannot live without it. Whenever birds fly, planes take off, and people speak, we experience and enjoy the visible effects and benefits of air. We may also experience the destructive forces of energy that result in tornadoes and hurricane-force winds. It is evident how beneficial and yet detrimental the invisible energy forces of air can be. In many ways it can be compared to electricity.
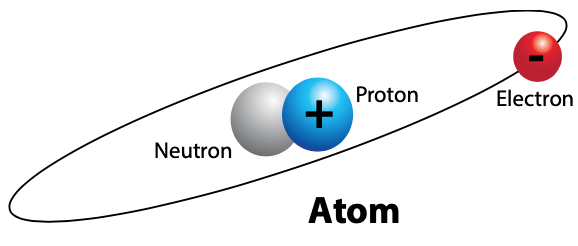
The existence, movement, or flow of electricity is a result of the invisible sub-atomic particles called electrons. When electrons move from atom to atom, this is called electrical current. Current is based on the measured quantity of electrons moving from one atom to the other with respect to time. With this understanding, students can now begin to focus on the dynamics of the electrons.
How does electricity come into being? According to the Bohr model, there are varieties of shells and sub-shells or rings of electron energy levels revolving around the nucleus of each atom. Each energy level may contain 1 to 2(n)2 electrons, where “n” equals the shell or ring number. Although the details of the atomic structure are interesting, it is more important for students to understand the significance of the furthest energy level or ring of electron(s) away from the nucleus of each atom. This energy level ring will be called the valence ring in this article, and this is the key to students understanding the concept of electricity, conductors, and insulators.
The valence ring of each atom is where understanding the nature of electricity often begins. If less than three electrons exist in the valence ring of any atom, these electrons can be easily influenced by natural forces to move away from one valence ring of an atom to the other. Therefore, when three or less electrons exist in the valence ring of any atom, these electrons are specifically called free electrons; free from nature’s chemical bond that holds them in the position of the valence ring. Electrons are said to move or travel at the speed of light and light speed is documented to be approximately 186,000 miles per second or 300,000,000 meters per second. To help students identify with such tremendous speed, think of being able to travel around planet Earth approximately 7.5 times in one second. This is how fast electrons are reported to travel. If five or more electrons exist in the valence ring of any atom, it becomes extremely difficult for any of the five or more electrons to be influenced and moved away from their valence ring to valence rings of other atoms.