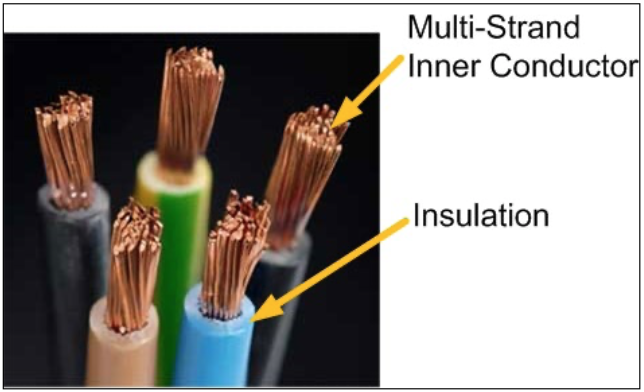
Electrical current is expressed in units of amperes (A). One ampere is said to be present when approximately 6.24 quintillion electrons (6,240,000,000,000,000,000) cross a specific point within the electrical pathway in one second. A quantity of 6.24 x 1018 electrons is said to be equivalent to one coulomb of charge. Research suggests that The International System of Units named this quantity of charge after Charles Augustin de Coulomb (1736-1806; French physicist). Although electrons are not visible to the naked eye, the effects of electricity can be realized by the presence of heat, light, electromagnetic force, effects of fire, sensation, and by measuring instruments such as digital volt ohmmeters (DVOM) and oscilloscopes. Rearranging electrons disproportionately within a structured device called a battery creates a potential voltage force in the same manner that stretching a rubber band creates a potential elastic force. The longer the length of the rubber band is stretched, the more potential elastic force (energy) will be released as the rubber band snaps back to its original resting position and form. Likewise, the more electrons that are disproportionately accumulated on one of the two conductive plate(s) within a battery, the more electrons will be available and naturally return to the vacant electron positions in the valence rings from where they were moved.
Electricity Made Simple - Part 2
Understand The Principles and Concepts of Electricity
Make sure to read Part 1 from April's Blog post.
When a stretched rubber band is released, its elasticity contracts as it snaps back to its original position. The force of the contraction is proportion-ate to the length and force of the stretch. If the same rubber band is stretched and released beneath water, the rubber band will experience a slower contraction rate as it snaps back to its original resting position. Contraction rate is now compromised due to the opposing force offered by the presence and substance of water. The water will offer opposition to the rubber band’s contraction rate. If the same process is repeated with the rubber band submerged in a thicker gel-like substance, the rubber band’s contraction rate will experience additional opposing forces from the gel-like substance. The thicker the gel-like substance, the more opposition or physical resistance to the rubber band effect.
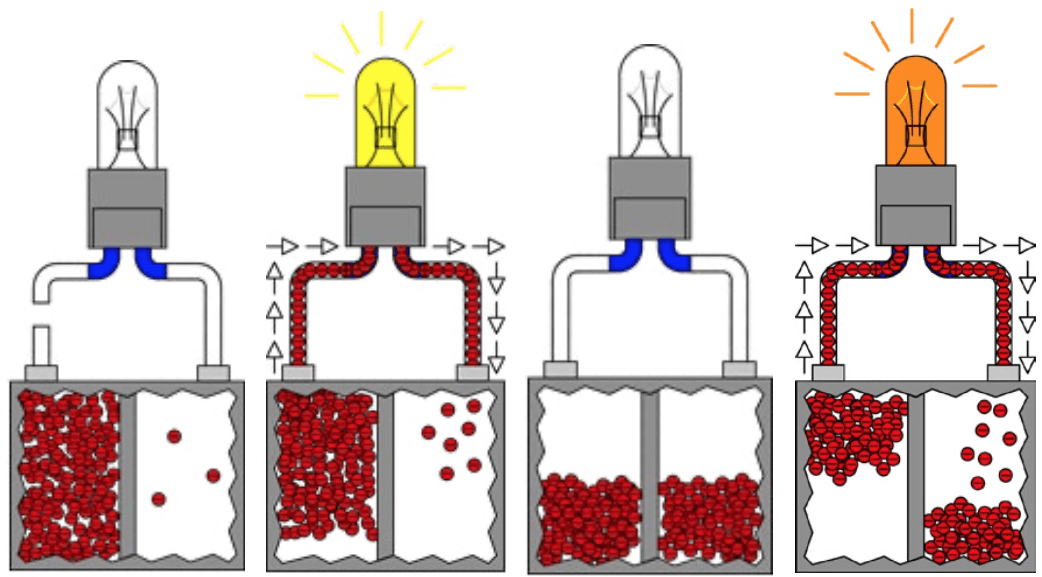
By explaining the basic concepts of electricity and providing real-life examples involving the dynamics of electricity, students begin to connect the dots and under-stand what electricity is really all about; what allows a battery to be fully charged and what causes a battery to be discharged. The distinction between charged batteries versus dead batteries is comparable to a stretched rubber band versus a rubber band that is not stretched. Stretching the rubber band awakens the potential elastic force in the rubber band material while displacing and accumulating electrons disproportionately establishes a potential voltage charge within a battery. There is no potential energy in a rubber band when it is not stretched. There is no potential voltage between atoms in a state of equilibrium. Natural laws, and the laws of physics and chemistry, allow for science to use natural principles of chemistry to cause electrons to accumulate disproportionately between two conductive plate(s). The difference between the number of electrons on the negative plate(s) and the positive plate(s) generates an electrical charge within the battery. Whenever any two different conductive materials are placed into an acidic solution or substance called electrolyte, a natural chemical reaction will occur. This chemical reaction causes electrons to move and accumulate disproportionately on one of the two conductive plates more so than the other. The plate that accumulates excessive electrons is considered to be the negative plate(s) or terminal of the battery and the conductive plate(s) that loses or is deprived of the equivalent number of electrons is considered to be the positive plate(s) or terminal of the battery. The chemical reaction enables the electrons to leave the plate(s) of one terminal and accumulate on the plate(s) of the other terminal.
Students can see and experience the concept of a battery by inserting two different types of conductive material into an acidic solution; i.e. a brass screw and a galvanized screw can be used as plates/terminals inserted into an acidic solution (body of electrolyte) such as a lemon or a potato. Students can use a DVOM to measure the potential voltage of approximately 600 to 1,000 millivolts (mV) that will develop across the brass and galvanized screw terminals of the lemon/potato-based battery. They can connect a light emitting diode (LED) to the correct polarity on the terminals of the lemon/ potato battery and see the LED light as the electrons flow from the lemon/potato battery. Students are always fascinated by live demonstrations. They get the opportunity to witness and experience the most fundamental electrical charge emanating from a fruit or vegetable. The voltage generated will primarily depend on the size and type of conductive screws or plates used and the specific gravity of the acidic solution as compared to water. One of the two screws will become the positive terminal and the other will become the negative terminal. Students can use a DVOM to verify which screw became the negative or positive terminal of the lemon/potato battery. The test leads of the DVOM can be switched between both screw terminals until a negative sign appears on the screen of the DVOM. This will identify the polarity of the screw terminals of the lemon/potato battery. Students will learn the significance of polarity. If a conductive pathway is provided to allow all displaced and accumulated electrons to return to their original destination, the battery will then be considered discharged or dead. Electrons will flow or travel from the negative terminal to the positive terminal via any electrical conductive pathway. When electrons flow from the negative to the positive terminal of the battery, this is called the electron flow. The conventional flow is based on the assumption that electrons flow from the positive terminal to the negative terminal of the battery. Should a higher voltage be required, several lemon/potato batteries can be connected in series; positive screw terminals connected to the negative screw terminals. This will cause the total voltage to double or triple for each lemon/potato battery connected in series.
Recharging a dead automotive battery is accomplished by using an external electrical source called a battery charger. Recharging a battery is comparable to stretching a rubber band again and again. When free electrons are pushed away from the valence rings of the original atom on the positive plate(s) and collected disproportionately on the negative plate(s) of the battery, the battery is re-charged. Potential elastic force is established in a rubber band, and potential voltage is established between the terminals of a battery. Unfortunately, after stretching a rubber band repeatedly, it will eventually fail. Likewise, after recharging an automotive battery repeatedly, the battery will also fail. Students will be able to connect these demonstrations and experiences to what they have learned.
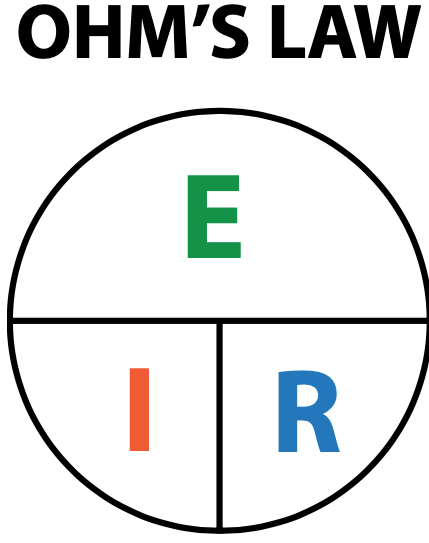
It is very important for students to understand the concept of electrical power. Electrical power ratings deter-mine how much electrical work a device or component can deliver or withstand before overheating or approaching catastrophic failure. The purpose for addressing power is not to explore the concept of electrical power at this time but rather to remind students of its importance. Electrical wattage is a critical determining factor in stereo systems, electrical motors, electrical drivers, and electrical bulbs. Adequate cooling and ventilation must be considered and provided for the durability of electrical devices and components. For example, excessive cranking of an automotive engine can cause the starter motor to burn out due to excessive heat and power consumption. Alternators must also be able to withstand the electrical power demands for all electrical systems on automotive vehicles. Resistors can be used to demonstrate the concept of electrical power by using three resistors of equal resistor value. However, the resistors should have different power ratings. One should be rated higher than the product of the current and volt-age they are connected to. The other should be half the power rating, and the third one should be less than half the required rating. Students can build a basic circuit and connect the resistors to a secured and stable power sup-ply that will provide the same voltage and current for all three resistors. Students can then observe and experience how the lowest power-rated resistor may literally burn while the highest-rated power resistor remains at room temperature. Despite the current and voltage remaining the same for all three resistors, the power rating of the resistors make an enormous difference in the outcome.